FREE Shipping on Orders over $89 with Account – Create One Today!
- (844)-859-9400
- Get Help


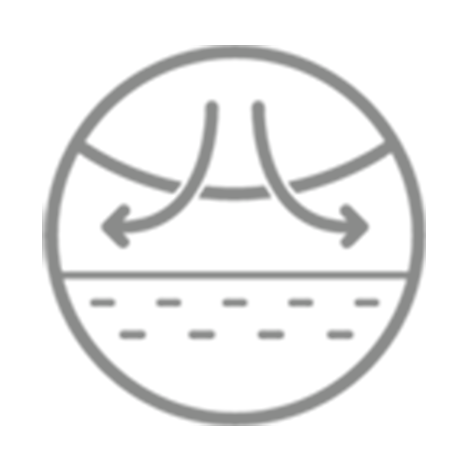



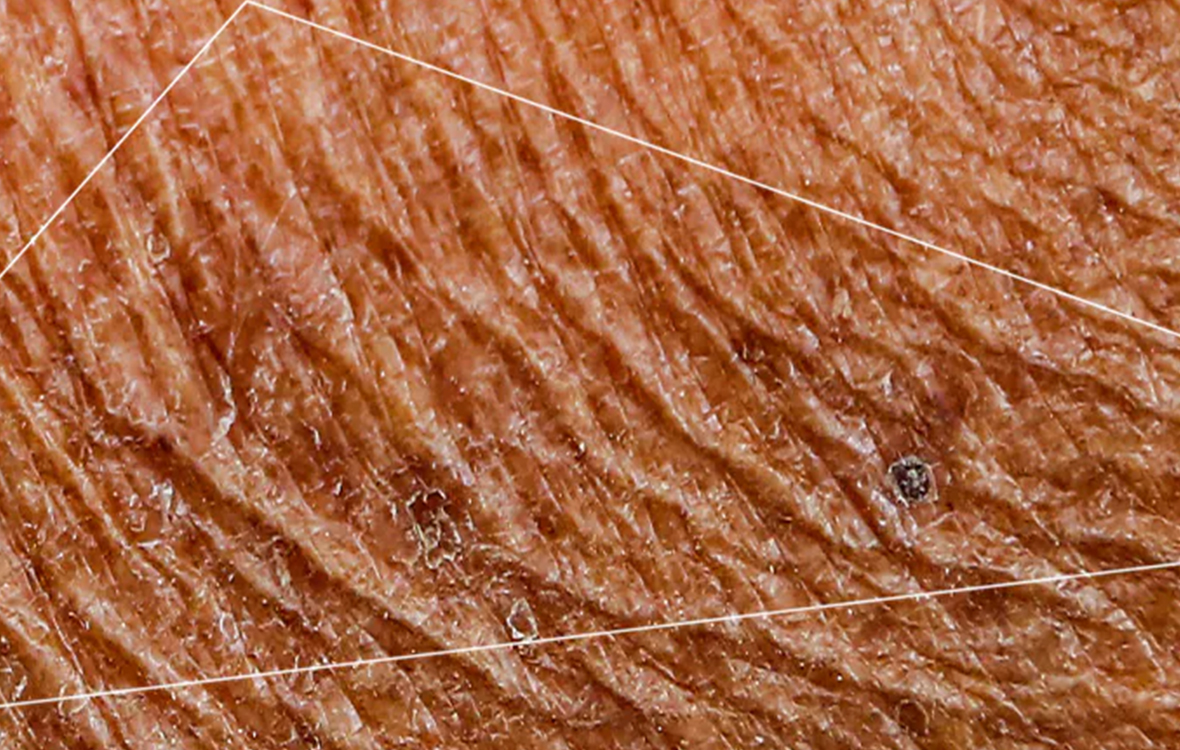

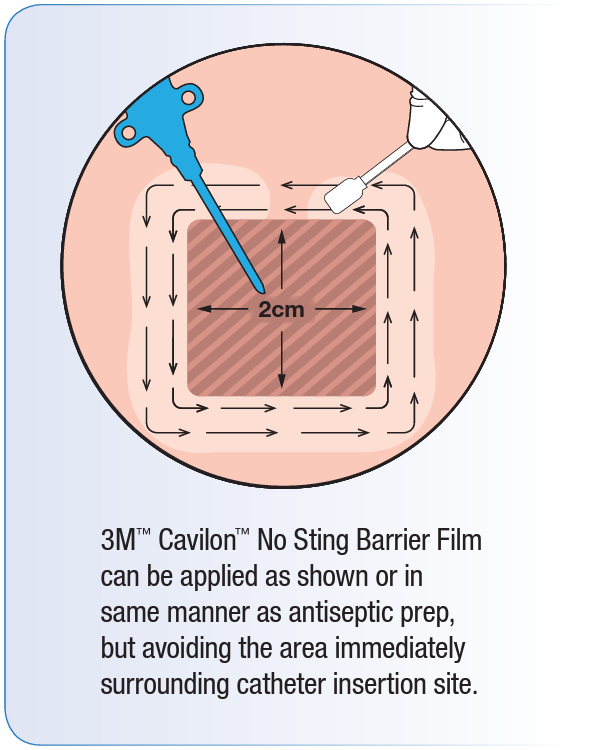
NOTE! Cavilon No Sting Barrier Film forms a protective interface between skin and the adhesive. It is removed with the adhesive product and must be reapplied with each dressing change.
